The Provention Bio Laboratory, based in Red Bank, New Jersey (USA), founded by Leon Immunologist Francisco León Prieto, has obtained the FDA license (United States Medicine Agency) for biological therapy, intravenous antibody,As the first and only immunomodulatory treatment to delay the appearance of type 1 diabetes in stage 3 in adults and pediatric patients from 8 years with stage 2. This is the first therapy approved for the prevention of type 1 diabetes.
The Biopharmaceutical company Prevention Bio was founded in 2016 by Francisco León - of which he is currently Executive Scientific Director - and Ashleight Palmer - current CEO.The laboratory is dedicated to intercepting and preventing autoimmune diseases, including celiac disease and myocarditis.
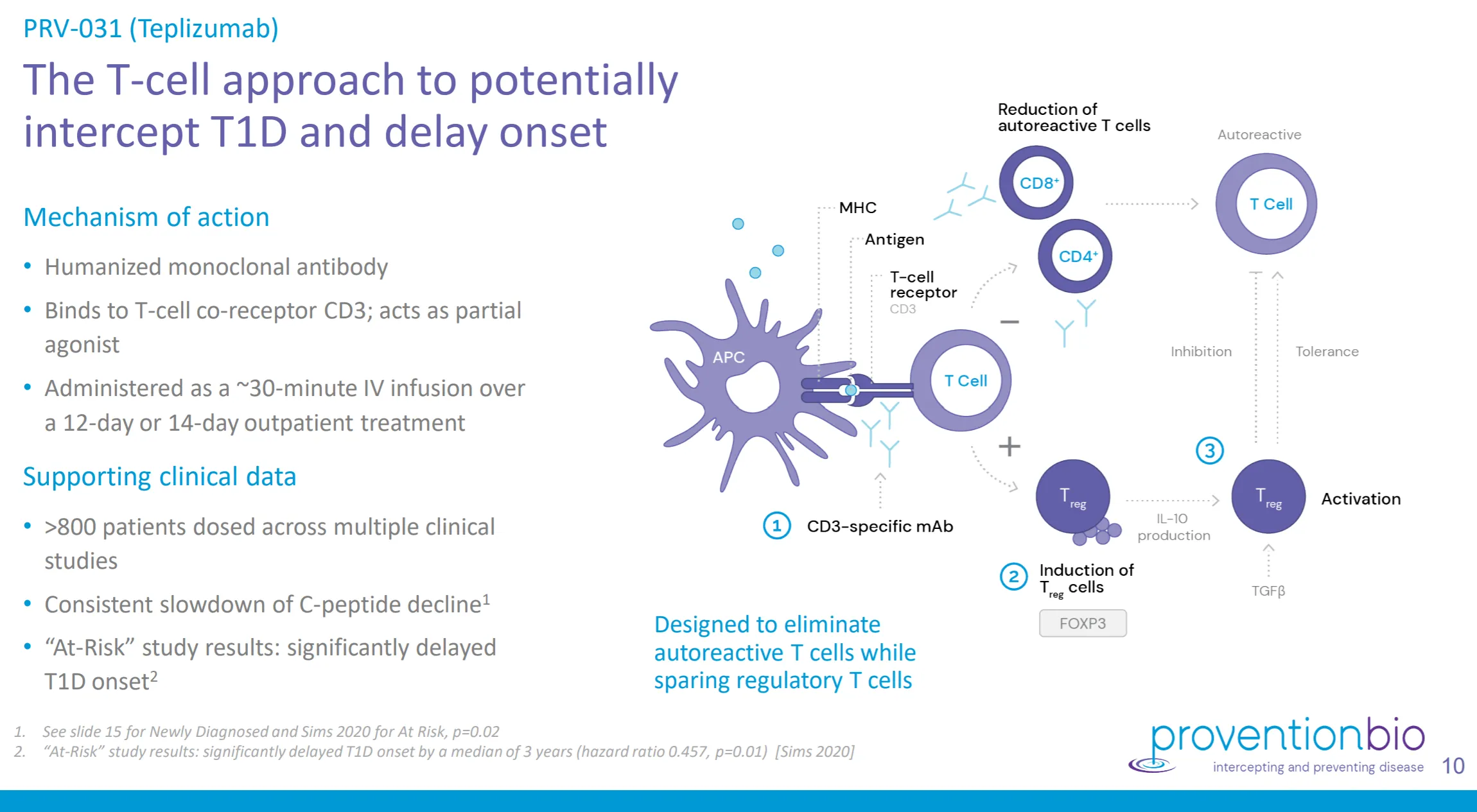
The Teplizumab monoclonal antibody, which will be marketed under the Tzield brand, of Provention Bio and Sanofi, is a vaccine that is administered intravenously.The medicine directs the immune system its own insulin producing cells.
At the moment, the therapy will be marketed in the United States, although Francisco León hopes that he will soon reach Spanish health."We are studying the best way to bring it to Europe and Spain, without a specific date but as soon as possible," he says from New Jersey.
the effort
"It is a satisfaction to see that we can help avoid thousands of people a disease"
For this Leonese entrepreneur "it is a huge satisfaction to see that we can finally help avoid a chronic self -limm disease to thousands of people."
In clinical trials, Tzield delayed diabetes progression in a little more than two years.According to the company, the benefits have lasted much more in some of the study participants.
The CNN news chain has disseminated the information in the United States after the approval of the FDA with the testimony of several people participating in clinical trials.One of them, Mikayla Olsten, was examined to detect diabetes after her 9 -year -old Mia sister, suddenly develops a potentially deadly episode of diabetic ketoacidosis and diagnose diabetes.
There was no history of diabetes in the family and Mikayla was not sick, but he had four of the five types of autoantibodies that doctors are looking for to evaluate a person's risk."They told us that when someone has so many markers, the doubt is not whether to develop diabetes, but when," said his mother, Tracy.Mikayla was 15 when he joined the studio and received Teplizumab.He is now 21 years old and is in the last year of the University.It undergoes a series of annual tests to review its pancreas and blood markers, and Tracy Olsten says that its diabetes has not progressed in six years.
arrival in Europe
"We are studying the best way to bring it to Europe and Spain, as soon as possible"
According to an investigation by the endocrine society and the American Diabetes Association, when a person has markers of autoimmune disease and episodes of sugar in uncontrolled blood, the risk of five years of progression to an insulin -dependent symptomatic disease is 75 %.The life risk of developing insulin -dependent diabetes is almost 100%.
«This is a historical occasion for the Type 1 diabetes community and a paradigm shift.For people 8 years or older in stage 2 who now have a therapy approved by the FDA to delay the appearance of the disease in stage 3, a delay in the beginning of stage 3 that gives perspective to the patient and family;More time to live without diabetes, ”says Ashleight Palmer, co -founder and Executive Director of Prevention Bio.
The main challenge to prescribeTzield will be to find people who need it.The medicine is approved for people who have no symptoms of the disease and they may not know that they are on their way to get it."Detection becomes a really important problem, because what we know is that about 85% of type 1 diagnoses are currently found in families that have no known family history."
use
Tzield is approved for use in people over 8 years of age who are in stage 2 of their type 1 diabetes. At that stage, doctors can measure the antibodies that attack the beta cells of insulin producers in the person's blood,And they have abnormal blood sugar levels, but their body can still cause insulin.
"The way not only the industry, but also our medical system handle autoimmune diseases, and especially type 1 diabetes, is really suboptimal in the current era," says Ashleight Palmer.
In October 2022, the company announced a copromotion agreement for the US launch of Tzield.The head of Sanofi consider that this approval is a "deep and long -awaited victory for the diabetic community" and agraced to Provention Bio "for its unwavering determination to bring to patients the first modifying therapy of the disease.We hope to take advantage of the infrastructure and experience of Sanofi to help people need it in the US ».
arrival in Europe
"We are studying the best way to bring it to Europe and Spain, as soon as possible"
The treatment is administered once a day for two weeks.The injection is supplied sterile, without preservatives, transparent and colorless.The most common side effects informed were white blood cells and low lymphatic cells, rash and headache.
In clinical trials, the treatment has stopped the disease before the symptoms appear, stopping the destruction of beta cells.The treatment restarts the immune system.
Unlike type 2 diabetes, which can be prevented with changes in lifestyle, such as losing weight and exercising, type 1 is a genetic disease that has not had prevention options so far.Until now.
The Leon participated in September in León in the 43th Congress of the Spanish Society of Immunology, which brought together five hundred scientific.Francisco León has collaborated with the León Hospital team in rehearsals on celiac disease.