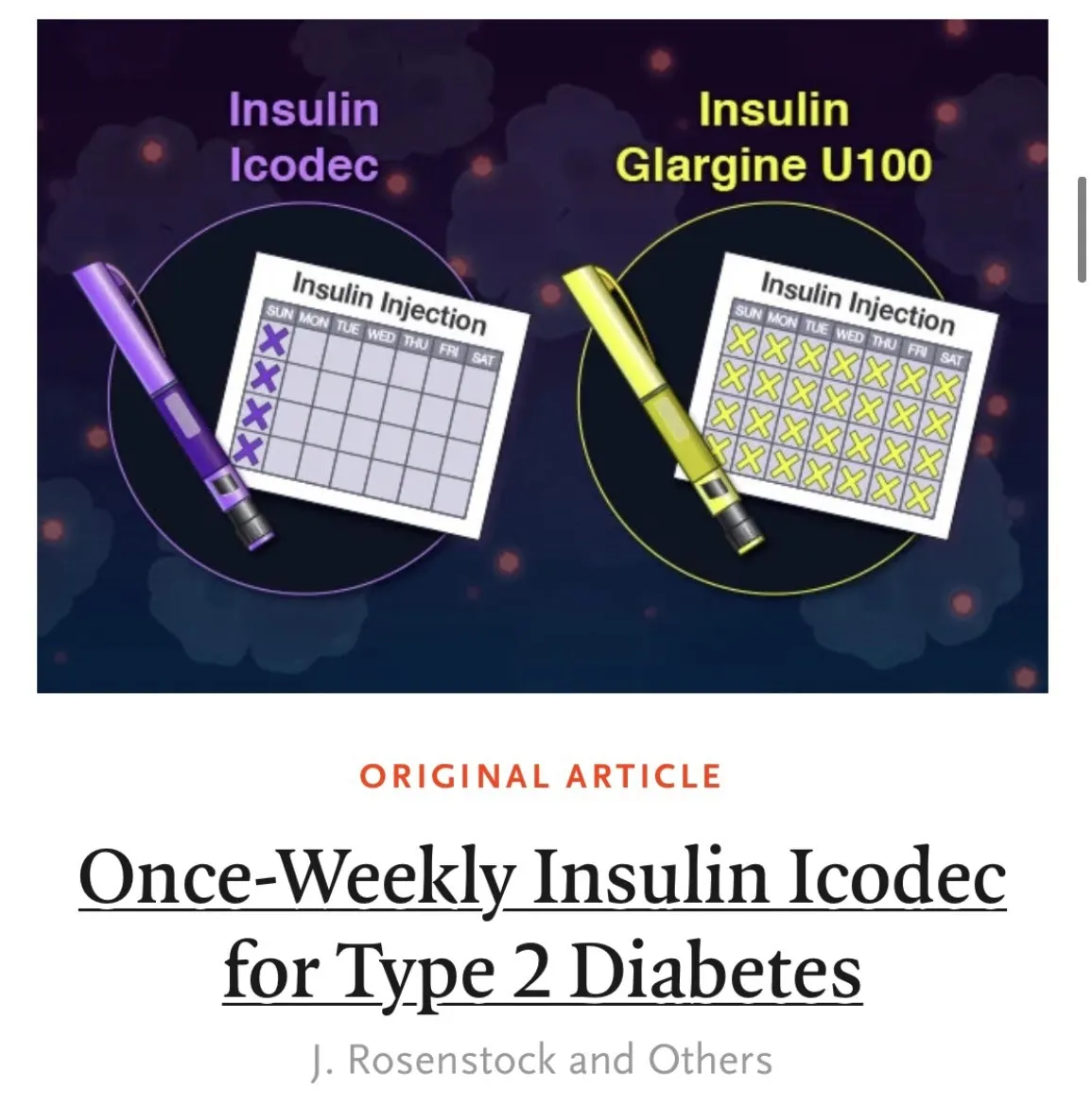
During the 58th Annual Meeting of the European Association for the Study of Diabetes (EASD), which is held in the Swedish city of Stockholm, the Novo Nordisk Pharmaceutical Company has presented new test data in phase 3a onwards 2, in thewhich shows that 37 percent of adults with type 2 diabetes treated with ICODECC insulin, of weekly administration, reached an HBA1C (glycosylated hemoglobin) of the & LT; 7 percent, without experiencing severe or clinically significant hypoglycemia, compared to 27percent of swallowing insulin treaties at 26 weeks follow -up.
In the words of the Head of the Department of Endocrinology and Nutrition of Madrid Hospitals Quirónsalud Pozuelo, Ruber Juan Bravo and San José, Dr. Esteban Jódar, "this weekly insulin is a great technological advance with a very positive impact on the patient's quality of life, in addition to the good results obtained compared to Insulin Degludec.
"Without a doubt, an important step forward in the treatment of diabetes that will require an educational effort by both people with diabetes and health professionals," said Esteban Jódar.
adaptation
In the opinion of this specialist, "moving from a daily insulin to a weekly may seem easy, but, like any change, it requires an adaptation period. Although I am sure that, in view of the good results in the studies, it will soon be areality in the day to day of our consultations and in the lives of people with diabetes. "
This essay achieved its main objective, showing non -inferiority in the reduction of HBA1C in week 26 with ICODEC insulin compared to swallowing insulin.From an average HBA of 8.17 percent (ICODEC) and 8.1 percent (swallow), ICODEC insulin, administered once a week, achieved a higher reduction in the estimated HBA1C of 0.93 byone hundred compared to 0.71 percent of the swallowing insulin.