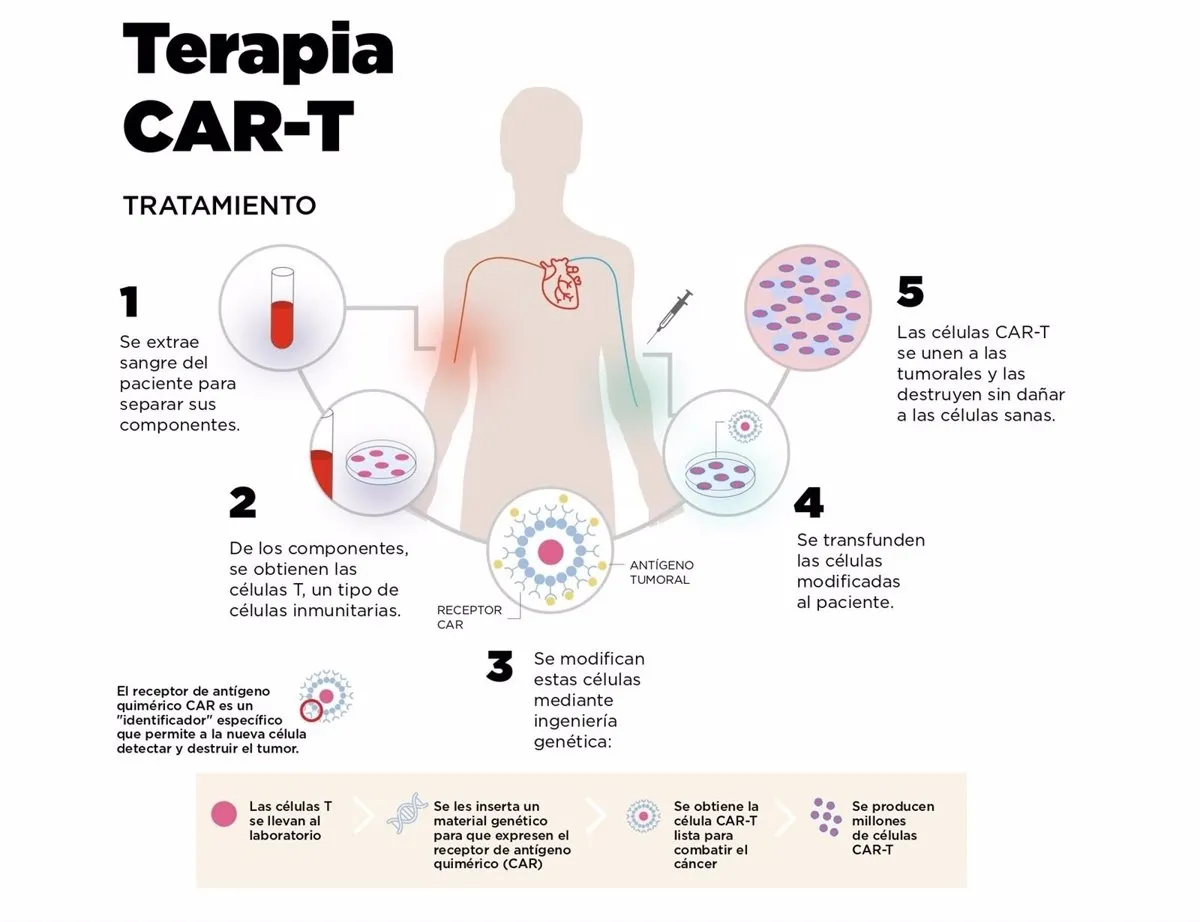
A study presented at the last meeting of the United States Endocrinology Society opens the door to a future elimination of insulin injections, the only viable treatment so far for type 1 diabetes. It is a therapy based on cells Car cells, that until now have shown tremendous success in leukemia and lymphomas.
These are cells of the immune system, known as T lymphocytes, which are genetically modified to recognize a specific antigen.In the case of hematological cancers, these are cytotoxic T cells, which recognize the tumor cell and destroy it.In diabetes, on the other hand, they focus on inflammation regulators.
Diabetes is an inability to regulate the presence of blood glucose related to a problem for insulin processing.In the case of type 2 diabetes, the most frequent, is almost always the result of the lifestyle (obesity, sedentary lifestyle), which ends up generating insulin resistance, vital to process the glucose present in the blood and that enters theCells
Type 1 diabetes, on the other hand, is an autoimmune disease caused by the destruction of beta cells of the pancreas, insulin producers, through T lymphocytes. It represents between 1% and 5% of all cases of diabetes, about 90,000 people in Spain according to the Spanish Diabetes Society.
It is usually diagnosed at an early age (4.9 years when detected in childhood) and 50% of patients have other associated pathologies, from the alteration of cholesterol levels to retinopathies and nephropathies.Although it is not related to the lifestyle, this does affect the development of the disease.
The team of Argentine Juan Carlos Jaume, professor of Endocrinology at the University of Toledo (Ohio, United States), obtained samples of blood and pancreas of patients who was extracted for some clinical reason (pancreas cancer and pancreatitis).From the blood obtained regulatory T cells, abbreviated as TREGS, which are responsible for regulating or suppressing other cells of the immune system, cultivated and genetically modified them to recognize antigens related to beta cells.
With this modification they introduced another: they associated with the edited T lymphocytes (car tregs) a green fluorescent protein.In this way they could verify if the cells, when they were introduced into the Langerhans islets (the pancreas structures produced by beta cells), survived and proliferated.
The researchers cultivated the pancreatic islets obtained from the human pancreas and combined them with the Car Tregs, which proliferated in the next 72 hours.They were also tested in modified mice to express human type 1 diabetes, showing the same behavior: these lymphocytes, genetically modified, suppressed the response of cytotoxic t lymphocytes that attack the beta cells of the pancreas, regenerating insulin production in the body.
the Cart revolution
"This is the next step of Car cells," explains the president of the Spanish Society of Immunology, Marcos López Hoyos."First have been hematological neoplasms (cancers) and, within a relatively short period, they will be used in solid organ tumors. Autoimmune diseases [such as type 1 diabetes] are the following."
In fact, there are already essays "in early stages" for lupus or multiple sclerosis, and also to avoid rejection in solid organ transplants."The concept of regulatory T cell was fashionable in the 80s between immunologists," he recalls, "but they fell into disuse and they were even proscribed," due to the difficulty in characterizing them molecularly.
Instead, effector T cells, which destroy cells,They were more successful."We can eliminate with cells everything we are eliminating with monoclonal antibodies", therapies that have supposed a step forward in the treatment of cancer in the last two decades.
However, López Hoyos does not believe that, if it exceeds all clinical trials in humans necessary to be approved, this therapy will be used a lot in type 1 diabetes, a well -controlled disease today thanks to innovations in the insulin administration, that make it more and more comfortable for patients.
"It can be a treatment route, above all, in cases considered rebels," says the immunologist.However, the study is another step to demonstrate the potential of a new way to treat numerous diseases.
"This therapy is in its beginnings," he says."The CARs have developed because, methodologically, we have applied many molecular tools and because in the last 20 years we have learned number of immune response mechanisms that we did not know before."Therefore, "in the next 20 years the change will be spectacular."