The use of an artificial pancreas is associated with a better control of blood sugar levels for people with type 1 diabetes compared to standard treatment, as a review of the available evidence published by 'The BMJ' has detected.
The results show that the artificial treatment of the pancreas provides almost two additional hours of normal blood glucose levels (Normaglycemia) per day, while reducing time both at high levels (hyperglycemia) and low (hypoglycemia) of blood glucose.
Although more research is needed to verify the findings, the authors affirm that these results support the opinion that "artificial pancreas systems are a safe and effective treatment approach for people with type 1 diabetes."
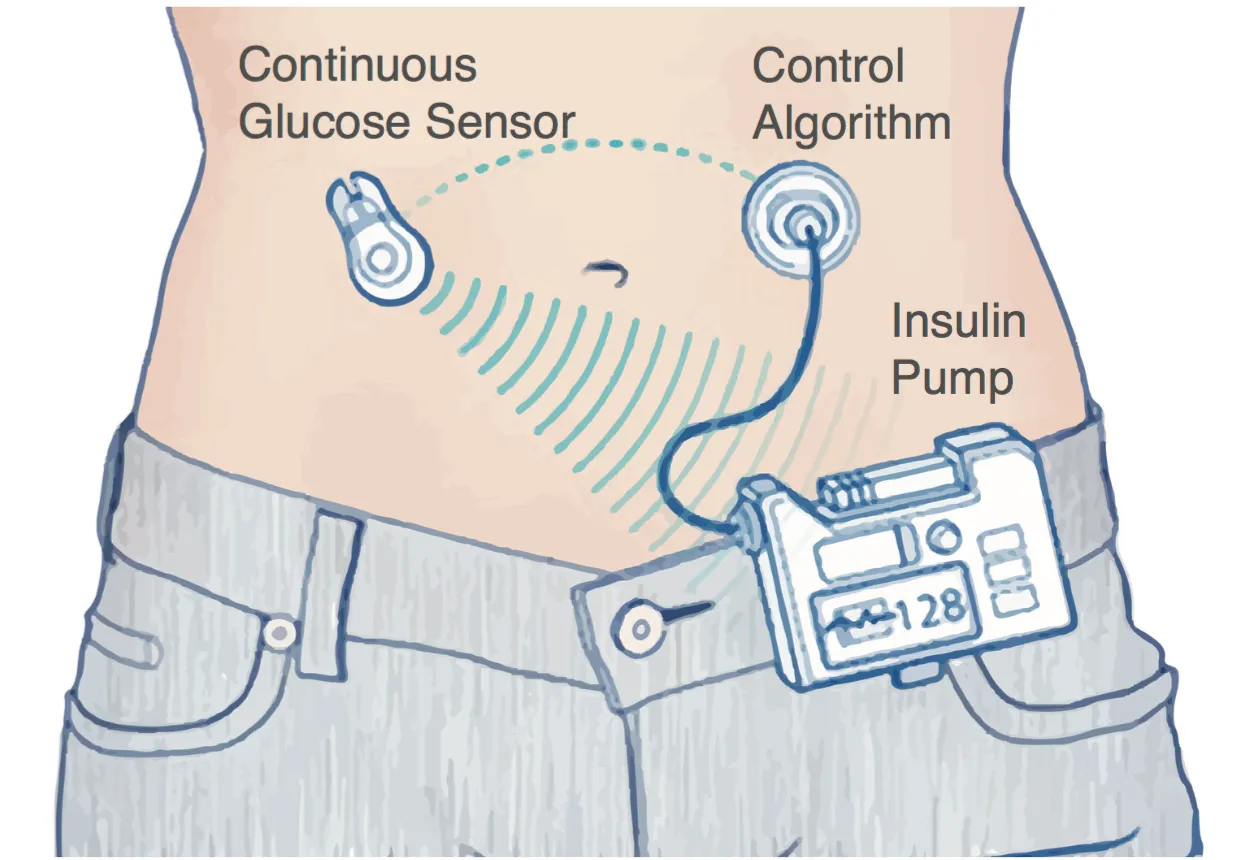
The artificial pancreas is a system that measures blood sugar levels using a continuous glucose monitor (MCG) and transmits this information to an insulin pump that calculates and releases the required amount of insulin in the body, as it doesThe pancreas in people without diabetes.The principal researcher, Eleni Bekiari of the Aristotle University of Bessaloniki, Greece, and the team set out to investigate the effectiveness and safety of artificial pancreas systems in people with type 1 diabetes.
These experts reviewed the results of 41 random controlled trials that involved more than 1,000 people with type 1 diabetes, which compared artificial pancreas systems with other types of insulin -based treatment, including insulin pump therapy.They found that the artificial pancreas is associated with almost two and a half hours additional in Normaglycemia compared to other types of treatment when used during the night and for a 24 -hour period.
Reduces hiccup times and hyperglycemia
The use of artificial pancreas also reduced the time elapsed in hyperglycemia in approximately two hours and hypoglycemia (20 minutes less) compared to other types of therapy.Additional analyzes to test the strength of associations for different devices and in different environments were consistent, suggesting that the results are solid.
As such, the authors say that their review provides a valid and updated description of the use of artificial pancreas systems for type 1 diabetes. However, they point out that most of the trials had a high or unclear bias risk, theSample size was small and the duration of the evaluation was short, so they must be interpreted with caution.
In addition, they suggest that more must be done to evaluate the cost-effectivity relationship "in order to support the adoption of artificial pancreas systems in clinical practice."The authors also recommend that future investigations "explore the artificial use of the pancreas in relevant groups of people with type 2 diabetes" and say that "the effect of the use of the artificial pancreas on the quality of life and the reduction of the patient's charge should be exploredfurther".