Johnson & Amp;Johnson continues with his long search for a cure of type 1 diabetes and has joined forces with the biotechnology firm to accelerate the development of the first stem cell treatment that could correct this hormonal disorder that puts life at risk.
Companies have already started tests in a small number of diabetic patients.If it works as well in patients as it has done in animals, it would be a cure for the disease and put an end to the need for frequent injections of insulin and blood sugar tests.
Viacyte and the Johnson & AMP group;Johnson Janssen Betalogics indicated on Thursday that they have agreed to combine their knowledge and hundreds of patents for their investigation through Viactye, J&Ap's partner; J for years and focused on regenerative medicine.
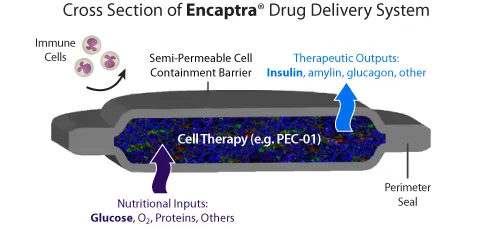
The therapy involves inducing embryonic stem cells to turn them into cells that produce insulin, into a small capsule that is implanted under the skin.The capsule protects immune system cells, which would otherwise attack them as invaders.This blockade has accelerated other research projects.
Also researchers from universities and other pharmaceuticals work towards a cure for diabetes using different strategies.But according to Viacyte and others, their treatment is the first tested in human patients.
If it succeeds, the product could be available in several years for patients of type 1, and over time could also treat type 2 diabetics that use insulin.
"This could be the good one," said Dr. Tom Donner, director of the Diabetes Center at the John Hopkins University School of Medicine."It's like manufacturing a new pancreas that manufactures all hormones" necessary to control blood sugar.
Donner, who does not participate in the study, said that if the device provides patients normal insulin levels, "it will prevent millions of diabetics from suffering dangerous complications."
People with type 1 diabetes no longer produce insulin, the hormone that converts blood sugar into energy, because their immune system has killed beta cells in the pancreas.These cells manufacture insulin in response to the rise in blood sugar levels after a meal.
Over the years, excess sugar in the bloodstream damages blood vessels and organs.Without effective treatment, diabetics suffer serious complications such as blindness, renal failure, heart problems, amputations and even premature death.On the other hand, too much insulin can be a very low level of sugar that can kill patients, especially young children.
According to the United States Diabetes Association, about 29.1 million Americans have diabetes, including 1.25 million with diabetes of type 1. The number of people with that variant of the disease, or dependent on insulin, grows in formcontinued.Meanwhile, the number of patients with type 2 diabetes, whose bodies manufacture insulin but do not use them efficiently, increases exponentially due to the global epidemic of obesity and sedentary lifestyle.
Many patients cannot control it well because the treatment is exhausting, requires a strict diet, frequent exercise, several daily insulin injections and other drugs, and several punctures on a finger to check the sugar level.In addition, some patients cannot pay the expensive medicines.
Viacyte Inc., based in San Diego, has been investigating its treatment for a decade, partly with financing from Juvenile Diabetes Research Fund and California Institute for Regenerative Medicine.
Johnson & Amp;Johnson, based in New Brunswick, New Jersey, is an important Viacyte investor and performsParallel investigations for about 13 years, said Diego Miralles, head of global innovation at J&CP J.
"We wanted to expand our bets to make sure we would win in this field ... that it is so transformational," Miralles said.
The manager declined to reveal the financial terms of the agreement with Viacyte.
The company began the first round of tests in patients a year ago its product, called VC-01, in a dozen people with type 1 diabetes, said Paul Laikind, executive director and managerial president of Viacyte.The subjects received a small amount of insulin producing cells on their devices, and will be monitored closely for two years to analyze insulin production and other effects.
After 12 weeks, the device had been properly attached to the nearby blood vessels, their new insulin producing cells continued to multiply and no side effects were identified.Soon, another dozen patients will receive the same dose of cells in capsules in an implant.
If everything goes well, in the next test round, a few patients will be implemented, they will receive the capsules with a complete dose of cells, probably in the second half of this year.They will take more evidence before regulators can approve the product.
"We believe it will have to be replaced periodically," said Laikind.
The previous tests carried out for years in thousands of mice showed that insulin producing cells matured and produced the necessary hormone in the mouse during the rodent's life, around one year, said Laikind.
Due to the protective capsule, which is flat and smaller than a business card, the device can immediately withdraw to avoid damage to the patient if something goes wrong.
The preliminary results are promising, Dr. Betul Hatipoglu, endocrine of the Cleveland clinic, said in an email.
"More research is needed to continue understanding its impact," he wrote, pointing out that researchers must refine the device precisely and determine if there are unforeseen security problems.
___
Linda A. Johnson is on Twitter as Link