This immunomodulatory drug would be able, in a subgroup of patients, to preserve the function of beta cells.Researchers announce broader studies and in combination with other therapies.
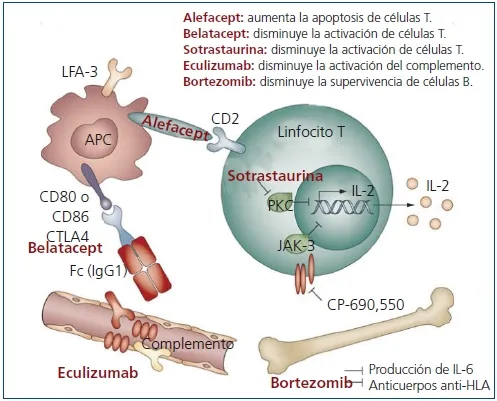
The administration of the Alefacept immunomodulatory drug (AMEVIVE, of Astellas), authorized by the FDA for psoriasis, would help preserve the functioning of beta cells for at least two years in patients with a recent diagnosis of type 1 diabetes mellitus (DM 1).
The Journal of Clinical Research magazine published last week positive results of the phase II T1dal study that, as Spanish experts explain to CF, open a hopeful route in the search for strategies to delay the evolution of DM1 and improve the prognosis of thedisease.
The importance of this effort is based on the fact that maintaining a level, even if it is modest, of endogenous insulin segregation brings significant long -term benefits.Future works will try to get more durable responses by combining Alefacept with other therapies, explains researcher Gerald Nepom, director of the Immune Tolerance Network (ITM) research consortium, sponsored by the National Health Institutes of the United States, which investigates strategies for disease preventionAutoimmune.
24 weeks of treatment
The T1dal, Double Blind and in front of Placebo trial recruited 49 patients between 12 and 35 years of those who 33 received Alefacept.The treatment, injectable, was administered weekly in two courses of twelve weeks with a break, between them, of another twelve.
In the treaties with Alefaccept, the C peptide index was reduced much less at the two years of therapy (0.134 NMOL/L on average for the Biological Group versus 0.368 NMOL/L for the placebo).In 9 of the 30 evaluable patients of the Alefaccept group, this index, marker of the preservation of beta cell, was not reduced at all, while this phenomenon only occurred in one of the 12 participants of the placebo.
The medicine also decreased insulin dependence (0.43 units/kilo/day vs 0.60 units/kilo/day), and severe hypoglycemia (see graphic), which reached 9.6 episodes/patient/patient/year frontat 19.1 of the placebo.It would be the first time that an immunomodulatory treatment would have reduced these episodes.Its adverse effects profile was also favorable and no serious toxicities associated with the drug were recorded.
Although "promising", experts consulted warn that it is a preliminary study."It is a scarce number of participants and it is still very difficult to predict what the future of this research will be," says Patricio Giraralt, endocrinologist and professor at the Faculty of Medicine of the University of Ciudad Real.One of the issues that should address coming studies will be to determine what clinical or immunological characteristics would be behind the great differences in the clinical response to the drug, says Ricardo García-Mayor, a researcher at the Galicia Sur Biomedical Foundation."In this study, only a subgroup responds, achieving a complete remission that would be equivalent to temporary healing."
Another issue that should be clarified would be its long -term clinical impact."Being preliminary results, the effect on the complications associated with diabetes is not clear," says Rebeca Reyes, coordinator of the Diabetes Group of the Spanish Society of Endocrinology and Nutrition (SEEN), for whom "it can be assumed"Lower incidence of hypoglycemia "would influence better glycemic control and a lower risk of complications."It would also be encouraging that the need for insulin decreases.
Alefacept acts in the face of the activation of T cells addressing the CD2 surface score.The blood samples of theParticipants revealed that it would selectively reduce proinflammatory T cells and preserve those that have protective properties.
previous studies
ITM researchers designed the study based on the works published in recent decades with immunomodulatory therapies of systemic and directed action.Some obtained a modest and transient preservation of beta cells, but many were manifest failures.
The intervention that would have reached the best clinical efficacy would have been the transplant of autologous hematopoietic tissue stem cells, although with a high price in complications.While many of these interventions would have brought relevant adverse effects, such as an increase in infections, García-Mayor points out that Alefacept could be free of these problems.