"The marketing authorization of 'Toujeo' is a very important milestone for Sanofi, since it allows usOnce, it reinforces our commitment to continue improving the quality of diabetes treatment, "said the senior vice president of the Global Diabetes Department of Sanofi, Pierre Chancel.
The European Commission's decision to grant the marketing authorization to 'Toujeo' in Europe is based on the results from the 'Edition' Clinical Development Program, a set of phase III studies carried out throughout the world that evaluated the effectiveness and effectiveness andSafety of 'toujeo' compared to 'Lantus' (insulin Glargina [DNR origin] for injection, 100 u/ml).They participated in more than 3500 adults with type 1 diabetes and type 2 without controlling with the treatment they received at that time.
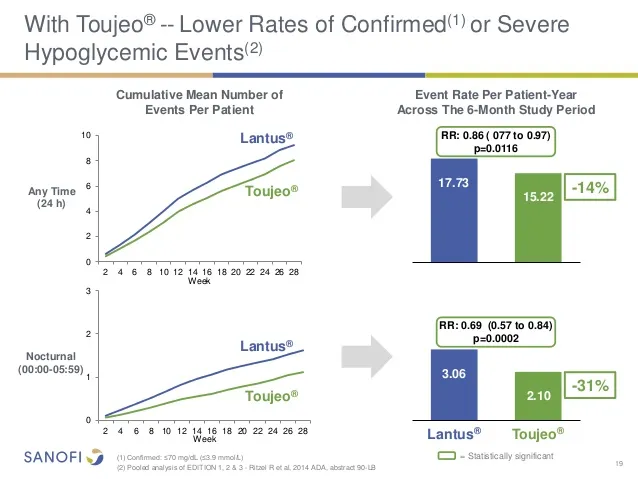
less hypoglycemia and a more stable glycemic control
The glycemic control with 'Toujeo' was similar to that of 'Lantus', with a favorable security profile.In addition, the incidence of confirmed hypoglycemia was lower with 'toujeo' than with 'Lantus', both insulins administered at any time of day or night, in people with type 2 diabetes. 'Toujeo' also demonstrated a more stable glycemic control andpredictable and a low intra -individual glycemic variability that lasts more than 24 hours compared to Lantus in people with type 1 diabetes.
"Many of the people who live with diabetes and who need insulin still failA new approach when addressing the united needs of the patients, "said the professor and head of the endocrinology, diabetology and pharmacodependence clinic of the Klinikum Schwabing, Städtisches Klinikum München GmbH, Munich (Germany), Robert Ritzel.
Toujeo's marketing authorization in Europe covers the 28 member states of the European Union, as well as Iceland, Liechtenstein and Norway, and is consonant with the favorable opinion issued by the Human Use Medicines Committee, of the European Agency of the European Agency ofMedications (EMA).In addition, the American drug agency (FDA) has approved 'Toujeo' and is in a review phase by other regulatory authorities of the world.