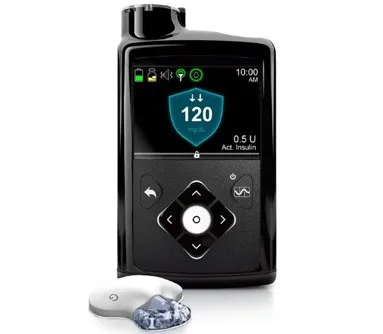
A few weeks ago, the entity responsible for protecting public health in the US approved Minimed 670G, the first portable "artificial pancreas" capable of controlling blood sugar levels of patients with diabetes 1 and administering the necessary amountof insulin automatically.
The device was developed by Medtronic, and basically consists of an insulin pump integrated by a closed circuit connection with a sensor that is responsible for measuring the person's glucose levels.
The 670G minimed represents an important advance with respect to the previous ones.
In the past, type 1 diabetics had access to insulin pumps, which are designed to administer insulin in preprogrammed doses to meet basal needs, while to determine blood sugar levels, the patient should use a monitor ofindependent glucose.
The Medtronic Minimed 670G, on the other hand, combines both the glucose sensor and insulin pump in a single device, facilitating its use by the patient.
This “closed circuit hybrid” system measures the user's blood sugar every five minutes, and then automatically injects the exact amount of insulin necessary to balance glucose levels.Also, an additional function allows the user to release insulin manually.
The Medtronic Minimed 670G, thanks to the fact that it includes smartguardian technology, also has the ability to automatically suspend insulin supply when a fall of chusemia is detected below the normal threshold, resuming the supply only after sugar levels areThey recover.
The recent approval of the Food & AMP;Drug Administration (FDA) was based on the positive results of a clinical trial that consisted of the use of the device in 123 type 1 diabetic patients.
It should be remembered that diabetes affects hundreds of millions of people worldwide.While the change in eating habits and physical exercise have proven to be good resources to improve the health of patients with type 2 diabetes, individuals with type 1 diabetes are unable to produce insulin at all and their symptoms are caused by deathof pancreas cells that are responsible for insulin production.
This last condition requires the constant supervision of blood sugar levels, which are maintained through regular insulin injections.Without access to medication, a type 1 diabetic cannot survive, hence the importance of having a system like the 670g minimed.
Tests to use the minimed 670g in children
For now, the use of the device has been authorized in people over 14 years, so the developer is dedicated to new trials aimed at testing its applicability in children from 7 to 14 years of age.
According to the information provided by Medtronic, the approval of the FDA occurred “long before expected”, so, taking into account this fact and the character of the applied technology, the Minimed 670G system will not be ready for itsDelivery to spring (boreal) of 2017.
Source: Medtronic